
USA: Which foods are potentially GMO?

Buy organic food!
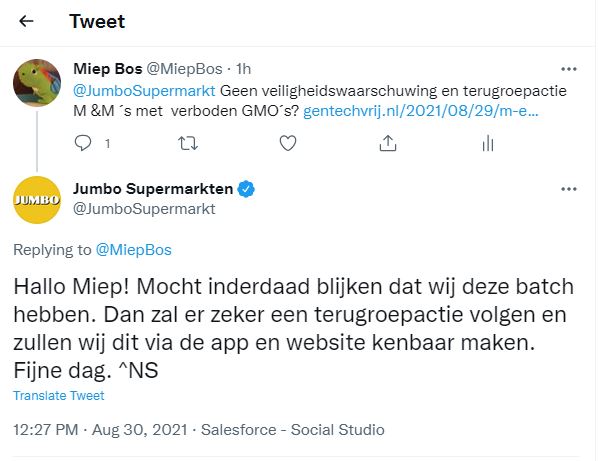
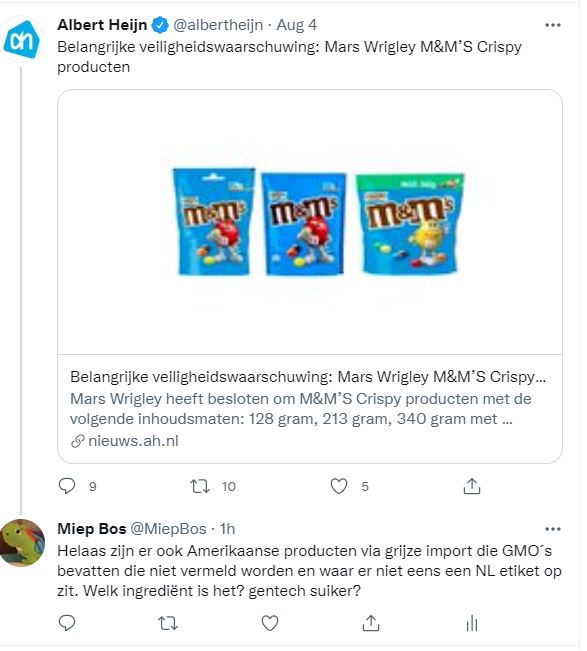
M en M terugroepactie in de EU

Bron: gmofreeusa.org In de EU wel een terugroepactie in de USA niet.
NVWA heeft gewaarschuwd: Mars heeft het op haar Nederlandse pagina gezet.
In the EU, Mars Wrigley recalled Crispy M&M’s due to the presence of a GMO. That’s right, the mere presence of a single, unlabeled, GMO ingredient caused the candy to be pulled off the market. https://kamcity.com/namnews/products-promotions/mars-recalls-some-mm-products-due-to-unauthorized-gmo-ingredient/…
Which M&M’s products were recalled? https://onestop.co.uk/wp-content/uploads/In-Store-Notice-MMs-Crispy-Recall-030821.pdf
Submit comments
Maize MON 87427 x MON 87460 x MON 89304 x 1507 x MON 87411 x 59122
Scientific opinion on application for authorisation of genetically modified Maize MON 87427 x MON 87460 x MON 89304 x 1507 x MON 87411 x 59122 for food and feed uses submitted under Regulation (EC) No 1829/2003 by Monsanto (EFSA-GMO-NL-2017-139)
EFSA opinion: 19 January 2021
Deadline: 21 February 2021
Lees verder “Submit comments”Open Consultations
Weer twee nieuwe toekomstige toelatingen van gentechmais tot de EU markt (geen teelt alleen voer en voedsel). Je kunt in je eigen taal reageren. Kies een vak en schrijf dat je het niet op je bord wilt hebben!
| Public consultations on GM food & feed authorisation applications | Food Safety (europa.eu)Maize Bt 11Scientific opinion on assessment of genetically modified maize Bt11 for renewal authorisation under Regulation (EC) No 1829/2003 (application EFSA-GMO-RX-016)EFSA opinion: 13 January 2021Deadline: 13 February 2021Submit comments |
| Maize 1507 x MON 810 x MIR162 x NK603Scientific opinion on application for authorisation of genetically modified 1507 x MON 810 x MIR162 x NK603 and subcombinations for food and feed uses submitted under Regulation (EC) No 1829/2003 by by Pioneer Hi-Bred International (EFSA-GMO-NL-2015-127)EFSA opinion: 13 January 2021Deadline: 13 February 2021Submit comments |
Bron EU
Open Consultations
| Maize MZIR098 Scientific opinion on application for authorisation of genetically modified Maize MZIR098 for food and feed uses submitted under Regulation (EC) No 1829/2003 by Syngenta (EFSA‐GMO‐DE‐2017‐142) EFSA opinion: 26 June 2020 Deadline: 27 July 2020 Submit comments |
EFSA opinion : “The GMO Panel does not identify safety concerns regarding the toxicity and allergenicity of the eC ry3.1Ab, mC ry3A (Bt´s!, webmaster) and PAT proteins (toxic herbicide glufosinate-ammonium! webmaster) as expressed in maize MZIR 098, and finds no evidence that the genetic modification would change the overall allergenicity of maize MZIR 098. In the context of this application, the consumption of food and feed from maize MZIR 098 does not represent a nutritional concern in humans and animals“.
De wetenschappers van EFSA zien geen enkele giftigheid bij deze gentech mais. Maar de herbiciden en de Bt die er over gesprayd worden dan? Daar doet de EFSA geen onderzoek naar! En dat al jaren! Schande! Dit moet stoppen! Bron/Source.
Fragmenten uit ons bezwaar:
Lees verder “Open Consultations”Bayer schikt bijna alle zaken rond giftige onkruidverdelger Monsanto
Fragement: “Bayer schikt voor een bedrag van maximaal 10,9 miljard dollar een serie rechtszaken in de VS rond de onkruidverdelger Roundup, die kankerverwekkende stoffen zou bevatten. Het chemieconcern heeft woensdag bekendgemaakt dat advocaten een akkoord hebben bereikt met bijna alle eisers.” Bron Nu.nl
Corporate Karma
Please share the video widely. Please share again and again.
A link to this video was contained in a joint press release (see below), between IRT and the German-based Coordination against BAYER-Dangers (CBG). On Thursday, April 23rd, our release went to about 700 European news outlets.
On Sunday, April 26th, CBD hosted Jeffrey at their online press event, which also featured a member of the European Parliament. Click here to view Jeffrey’s presentation.
Jeffrey also presented at the day-long online protest event that occurred in parallel with Bayer’s annual meeting on Tuesday.
He submitted two questions, with significant explanation, to be read during the shareholder meeting. Although Bayer claimed they read and answered every question during the meeting, they reduced his two minute explanation to a butchered 5 seconds. Bayer thereby depriving its shareholders and the public from hearing Jeffrey’s dire prediction about the company’s future, and specific remedies needed to help prevent bankruptcy.
What follows is the text of yesterday’s press release provides Jeffrey’s analysis and recommendations.
Lees verder “Corporate Karma”Weer een aanvraag voor een markttoelating van een gentech gewas, Maize MON 88017.
Open Consultations
| Maize MON 88017 EFSA Scientific Opinion on the assessment of genetically modified maize MON 88017 for renewal authorisation under Regulation (EC) No 1829/2003 EFSA opinion: 12 March 2020 Deadline: 13 April 2020 Add your comments |
(c) Name of the product (commercial and any other names)
MON 88017 maize was developed by Monsanto Company and provides protection
against certain coleopteran pests and tolerance to glyphosate based herbicides by the
expression of Cry3Bb1 and CP4 EPSPS proteins, respectively. It is associated with the
trademark YieldGard VT Rootworm/RR21. Gegevens van de fabrikant.
Risk assessment of genetically engineered plants that can persist and propagate in the environment
Paper Bauer-Panskus, A., Miyazaki, J., Kawall, K. et al. Risk assessment of genetically engineered plants that can persist and propagate in the environment. Environ Sci Eur 32, 32 (2020). https://doi.org/10.1186/s12302-020-00301-0
Fragment abstract:
Abstract
“New challenges arise in risk assessment when genetically engineered (GE) plants can persist and propagate in the environment as well as produce viable offspring. Next generation effects can be influenced by heterogeneous genetic backgrounds and unexpected effects can be triggered in interaction with environmental conditions. Consequently, the biological characteristics of the original events cannot be regarded as sufficient to conclude on hazards that may emerge in following generations. Potential hazards identified by the European Food Safety Authority (EFSA) include exacerbating weed problems, displacement and even extinction of native plant species.”
4-03-20 See also here (GMWatch) Spreading the risks: When genetically engineered organisms go wild.

